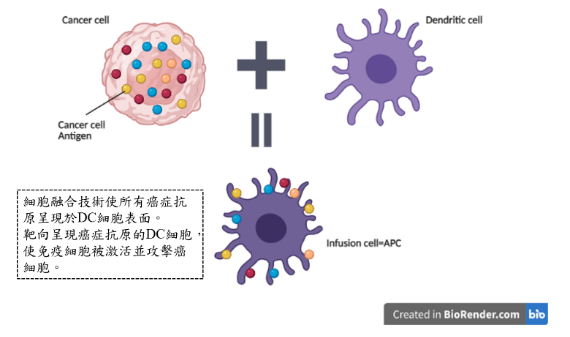
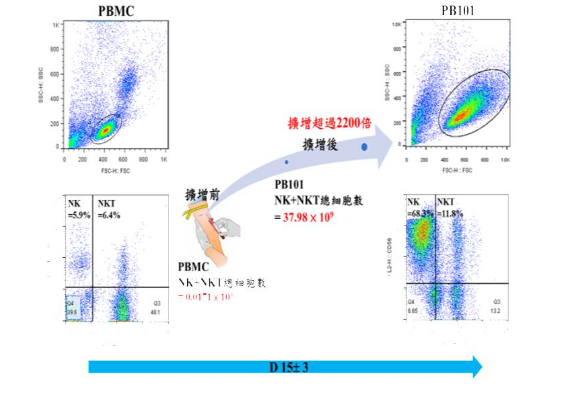
Precision Biotechnology passed the Taiwan Food and Drug Administration Clinical Trial Review (IND) in October 2018 and conducted Phase I clinical trials. It has experience in conducting human clinical trials and optimizing cell preparation processes. In cell preparation analysis, it can provide sub population segmentation of immune cells such as T cells (CD3, CD4, CD8, CD25), natural killer cells (CD16, CD56, NKG2D), and antigen presenting cells (CD80, CD86, CD40); It also provides analysis of immune activation and regulatory proteins related to cytokines.
In addition, in terms of cell product quality testing, we provide a platform for sterility testing in the Chinese Pharmacopoeia and testing the cytotoxicity of immune cell preparations against cancer cells.